The Families of Instruments Are Arranged From Top to Bottom in Order of
Prof. Liwayway Memije-Cruz is an author, educator and speaker. She teaches science subjects in different universities and colleges.
Periodic Tabular array
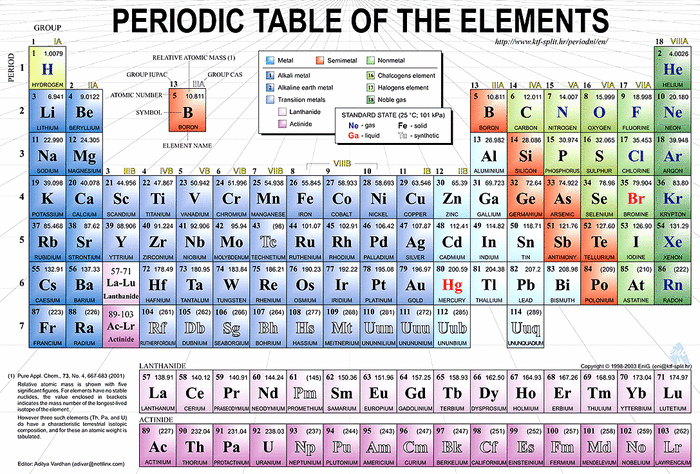
Periodic Table is the tabular arrangement of all the chemical elements which are organized based on diminutive numbers, electronic configurations and existing chemical properties.
Objectives:
Upon completion of this lesson, the students should be able to:
i. list the characteristics of the modern periodic table
2. classify the elements in the periodic table
3. explain the periodicity of elements
explain the periodicity of elements
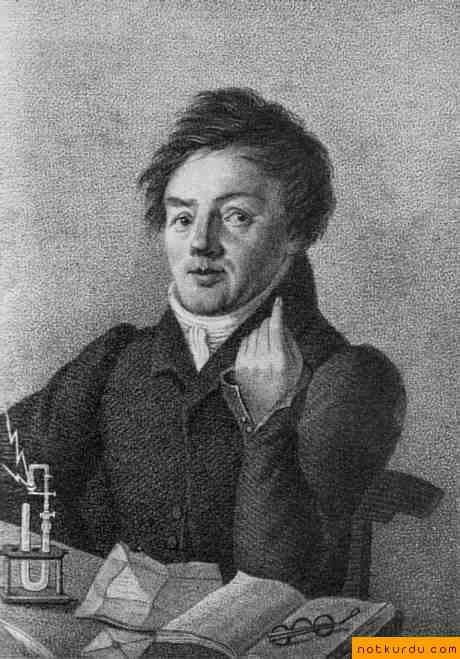
Johann Wolfgang Dobereiner classified the elements in groups of iii called triads.
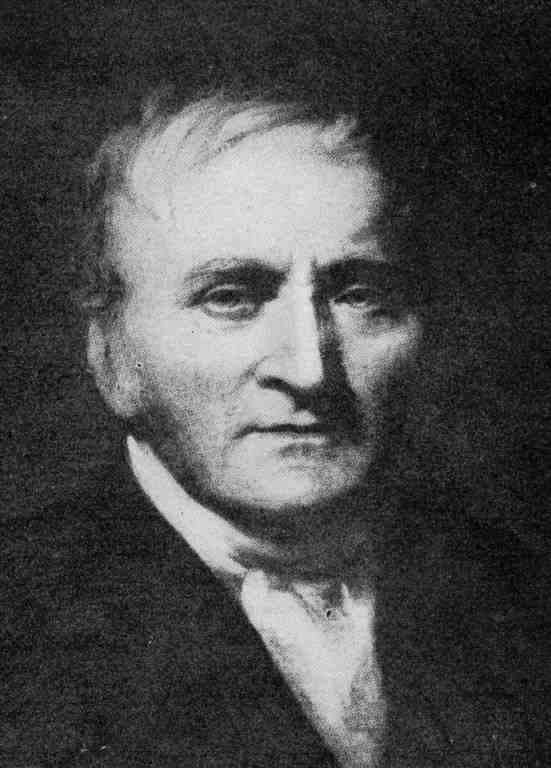
John A. Newlands arranged the elements in the order of increasing atomic mass.

Lothar Meyerplotted a graph showing an endeavour to grouping elements according to atomic weight.

Dmitri Mendeleev arranged in the social club of increasing atomic weights with a regular repetition (periodicity) of physical and chemical backdrop.

Henry Moseley is known for the Mod Periodic Law.
Evolution of Periodic Tabular array
As early as 1800, chemists began to make up one's mind the atomic weights of some elements with fair accuracy. Several attempts were made to classify the elements on this basis.
1. Johann Wolfgang Dobereiner (1829)
He classified the elements in groups of 3 called triads, based on similarities in backdrop and that the atomic mass of the middle member of the triad was approximately the boilerplate of the atomic masses of the lightest elements.
2. John A. New Lands (1863)
Read More From Owlcation
He arranged the elements in the order of increasing atomic mass. The eight elements starting from a given one is a kind of repetition of the beginning like the eight notes of the octave of music and chosen information technology the law of octaves.
3. Lothar Meyer
He plotted a graph showing an endeavor to grouping elements according to atomic weight.
4. Dmitri Mendeleyeev (1869)
He worked out a Periodic Table of Elements were the elements were arranged in the lodge of increasing atomic weights with a regular repetition (periodicity) of physical and chemical properties.
5. Henry Moseley (1887)
He arranged the elements in the order of increasing atomic numbers, which relates that the properties of the elements are periodic functions of their diminutive numbers. This is known as the Modernistic Periodic Law.
What are periods, groups and families?
Periods are the 7 horizontal rows in the periodic table
- Menses 1 has 2 elements respective to 2 electrons in the s sublevel.
- Periods ii and iii have 8 elements respective to viii sublevel electrons in the s and p sublevels.
- Periods 4 and 5 have 18 elements corresponding to xviii electrons in the s,p and d sublevels.
- Periods half-dozen and vii also include the 14 f electrons just the seventh menstruum is incomplete.
Groups are the vertical columns in the periodic table, which are divided into A, and B subgroups. The A subgroups are often called families . Some of the A families are designated by the following:
a. Group IA - Alkali Metals
b. Group IIA - Alkaline Earth Metals
c. Group VIIA - Halogens
d. Group VIIIA - Noble Gases
Other A subgroups are classified co-ordinate to the start element in the column:
a. Group IIIA - Boron Family
b. Grouping IVA - Carbon Family
c. Grouping VA - Nitrogen Family
d. Group VIA - Oxygen Family
Classification of Elements in the Periodic Table
i. Representative Elements are the elements in A Grouping/ Family. The term representative element is related to stepwise add-on of electrons to the southward and p sub levels of the atoms. Elements belonging to the same grouping or family have similar properties.
2. Noble Gases or Inert Gases are the elements in the terminal group with completely filled set of south and p orbitals.
three. Transition Elements are the elements in the columns IB - VIIIB which are chosen the B Group/Family. Accept note that they start with IIB up to VIIB, which have 3 columns and then end with IB and IIB. These sequences, which contain 10 elements each, are related to the stepwise addition of the x electrons to the d sub level of the atoms. These elements are metal-dense, lustrous, good conductor of estrus and electricity and in the most cases are hard. They grade the many colored compounds and course polyatomic ions similar Mn04 and CrO4.
4. Inner Transition Elements are the 2 additional horizontal rows below composed of 2 groups of elements which were discovered to have similar characteristics as Lanthanum in the 6thursday period called Lathanoids (Rare Earth Metals) and Actinium (Heavy Rare Elements). The Lanthanoids are all metals while the Actinoids are all radioactive. All the elements after Uranium are produced artificially past nuclear reactions.
The Periodic Table and Electronic Configuration
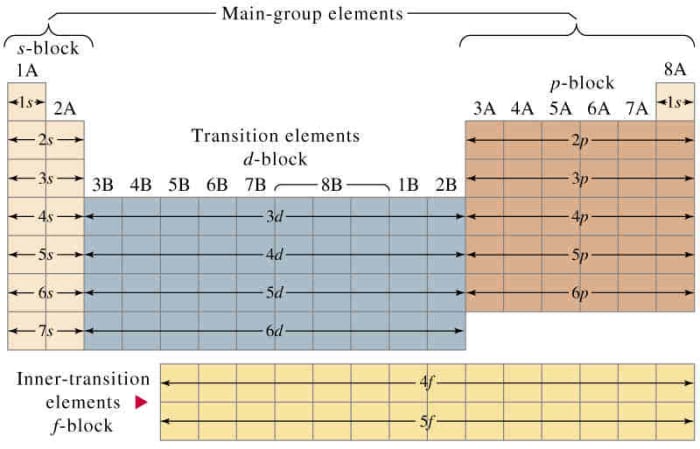
The ground land electronic configuration of the element is related to their positions in the Modernistic Periodic Table.
The Concept of Valence
Elements within any group exhibit a characteristic valence. The alkali metals of group IA exhibit a valence of +one, since the atoms hands lose the one electron in the outer level. The halogen of Group VIIA has a valence of -1, since one electron is readily taken up. In general, atoms, which accept less than four valence electron, tend to surrender electron thus having a positive valence corresponding to the number of electrons lost. While atoms with more 4 valence respective to the number of electrons gained.
Examples:
12 Mg 1s2 2s2 2p6 3s2
Magnesium will give up its two valence electrons forming a +2 valence
8 O 1s2 2s2 2p4
Oxygen has six valence electron thus it volition proceeds ii electrons -ii valence Grouping VIIIA has a stable outer configuration of electrons (with 8 valence electrons) and would not be expected to give up or take up electrons. Thus, this group has a zero valence.
In the B serial, the incomplete level contributes to valence characteristics. One or two electrons from an incomplete inner level may exist lost in chemical change and added to one or two electrons in the outer level, which allows possibilities of valence among the transition elements.
Examples:
26 Fe 1s2 2s2 2p6 3s2 3p6 4s2 3d6
Iron may exhibit valence of +two past loss of the two outer electrons or a valence of +iii when boosted electron is lost from the incomplete 3rd level.
Lewis Dot Organisation: Kernel Note and Electron Dot Notation
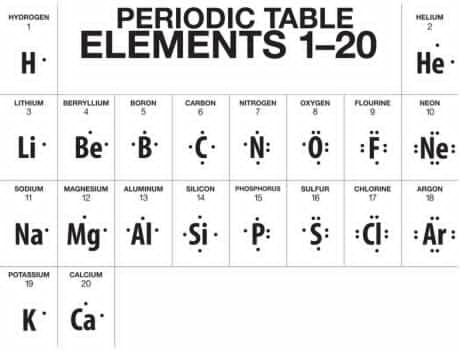
The kernel notation or electron dot notation is used to show the valence electrons in the atoms. The symbol of the elements is used to represent the nucleus and all inner electrons and dots are used for each of the valence electron.
Metals are at the left and in the middle of the Periodic Table. About 80 elements are classified as metals including some grade in every group except Groups VIIA and VIIIA. The atoms of metals tend to donate electrons.
Nonmetals are at the far right and toward the top of the Periodic Table. They are composed of about a dozen relatively mutual and important elements with the exception of Hydrogen. Atoms of non-metals tend to have electrons.
Metalloids or borderline elements are elements that to some extent exhibit both metallic and nonmetallic properties. They usually act as electron donor with metals and electron acceptor with not-metals. These elements prevarication in the zigzag line in the Periodic Table.
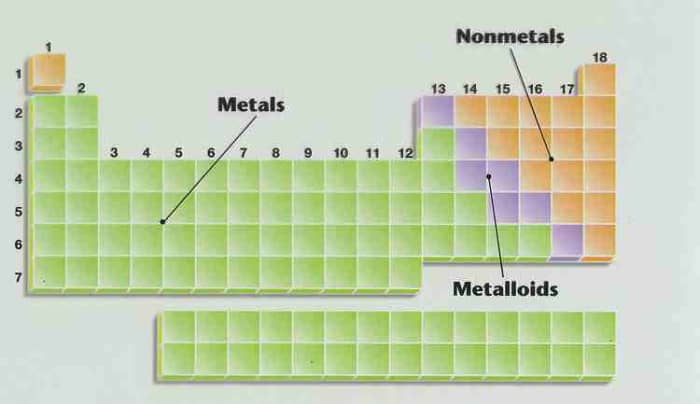
Metals, nonmetals and metalloids are neatly arranged in the Periodic Table.
Trends in the Periodic Table
Atomic Size
The atomic radius is approximately the distance of the outermost region of electron accuse density in an atom drops off with increasing distance from the nucleus and approaches zero at a large distance. Therefore, at that place is no sharply divers purlieus to decide the size of an isolated atom. The electron probability distribution is affected by neighboring atoms, hence, the size of an cantlet may modify from one condition to another every bit in the formation of compounds, nether different weather condition. The size of the atomic radius is adamant on covalently bonded particles of elements as they exist in nature or are in covalently bonded compounds.
Going across any period in the Periodic Table, there is a decrease in the size of the diminutive radius. Going from left to right, the valence electron are all in the same free energy level or the aforementioned full general altitude from the nucleus and that their nuclear charge increased past one. Nuclear charge is the force of attraction being offered by the nucleus towards electrons. Therefore, the greater the number of protons, the greater is the nuclear charge and the greater is the over pull of the nucleaus on the electron.
Examples:
Consider the atoms of Period three:
Na 2e 8e 1e Mg 2e 8e 2e Al 2e 8e 3e
Consider the electronic configuration of Group IA elements:
Na 2e 8e 1e
K 2e 8e 8e 1e
Rb 2e 8e 18e 8e 1e
Cs 2e 8e 18e 18e 8e 1e
Fr 2e 8e 18e 18e 18e 8e 1e
Although the number of protons from top to bottom within the same group increases, the atomic size still increases due to the additional energy level equally seen from the in a higher place illustration. Therefore, atomic size increases from meridian to lesser within the same group.
Atomic size and Periodic Table
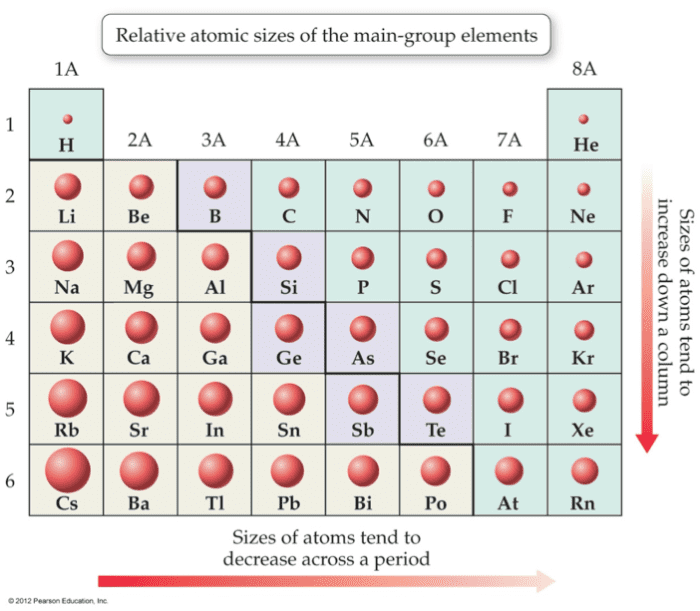
Atoms get smaller from left to right in a catamenia.
Ionic Size
When an atom losses or gains electron, it becomes a positively/negatively charge particle chosen ion.
Examples:
Magnesium losses 2 electrons and becomes Mg+2 ion.
Oxygen gains two electrons and becomes 0-2 ion.
The loss of electrons by a metallic atom results in a relatively large decrease in size, the radius of the ion formed is smaller than the radius of the atom from which it was formed. For nonmetals, when electrons are gained to class negative ions, in that location is a rather large increase in the size due to the repulsion of the electrons for one another.
Ionic size and Periodic Table
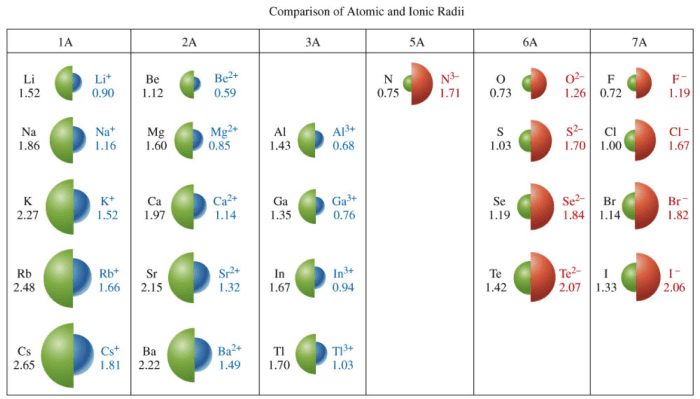
Cation and anion increase in size every bit you lot get down a grouping in a Periodic Table.
Ionization Energy
Ionization energy is the amount of energy required to remove the virtually loosely bound electron in a gaseous atom or ion to give a positive (+) particle of cation. The kickoff ionization energy of an atom is the amount of energy required to remove the first valence electron from that cantlet. The 2nd ionization energy of an atom is the amount of energy required to remove the 2d valence electron from the ion and so forth. The 2nd ionization free energy is e'er higher than the first, since an electron is removed from a positive ion, and the tertiary is also higher than the second.
Going across a catamenia, there is an increase in the ionization energy due to the removal of electron in each case is at the aforementioned level and there is a greater nuclear charge belongings the electron.
Factors affecting the magnitude of the ionization potential:
- The accuse of the atomic nucleus for atoms of similar electronic arrangement. The greater the nuclear charge, the greater the ionization potential.
- The shielding effect of inner electrons. The greater the shielding effect, the smaller the ionization potential.
- The diminutive radius. As the diminutive size decreases in atoms with the same number of free energy levels, the ionization potential increases.
- The extent which the nigh loosely spring electron penetrates the cloud of inner electrons. The caste of penetration of electrons in a given main energy level decreases in the order of s>p>d>f. All other factors being equal, every bit in the given atom, it is harder to remove an (s) electron than a (p) electron, a p electron is harder than a (d) electron, and d electron is harder than an (f) electron.
Bonny forcefulness between the outer level electrons and the nucleus increases in proportion to the positive charge on the nucleus and decreases with respect to the distance separating the oppositely charged bodies. Outer electrons are not but attracted by the positive nucleus but are also repelled by electrons in the lower free energy levels and their own level. This repulsion, which has the internet upshot of reducing the affective nuclear charge, is chosen the shielding effect or screening issue. Since from top to lesser, ionization energy decreases in A family, the screening effect and distance factors must outweigh the importance of the increased accuse of the nucleus.
Ionization Energy and Periodic Table
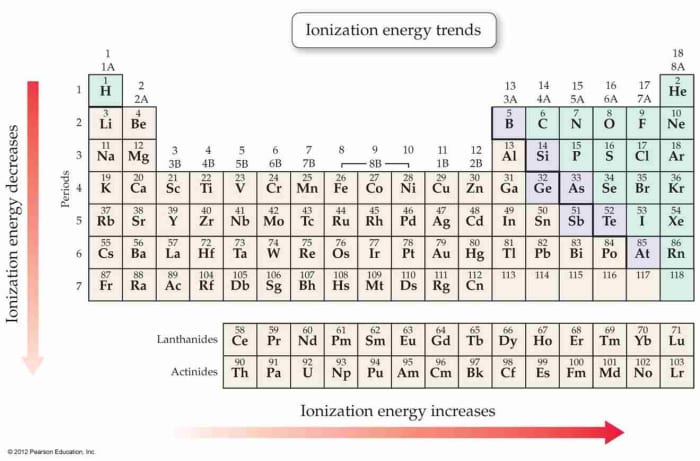
Going across a period, there is an increase in the ionization energy due to the removal of electron in each instance is at the aforementioned level and there is a greater nuclear charge holding the electron.
Electron Affinity
Electron analogousness is the energy given off when a neutral gaseous atom or ion takes in an electron. Negative ions or anions are formed. Determining electron affinities is a hard job; only those for the most nonmetallic elements have been evaluated. A second electron affinity values would involve gain and not loss of energy. An electron added to a negative ion would result in Coulombic repulsion.
Case:
0 + due east- -------------- 0-1 -33 Kcal/mole
0 + e- -------------- 0-2 +189 Kcal/mole
This periodic trends of electron analogousness, of the strongest nonmetals, the halogens, are due to their electron configuration, ns2 np5 that lack a p orbital to accept stable gas configuration. Nonmetals tend to proceeds electrons to class negative ions than metals. Group VIIA has the highest electron affinity since merely one electron is needed to complete a stable outer configuration of 8 electrons.
Electron Analogousness and Periodic Tabular array
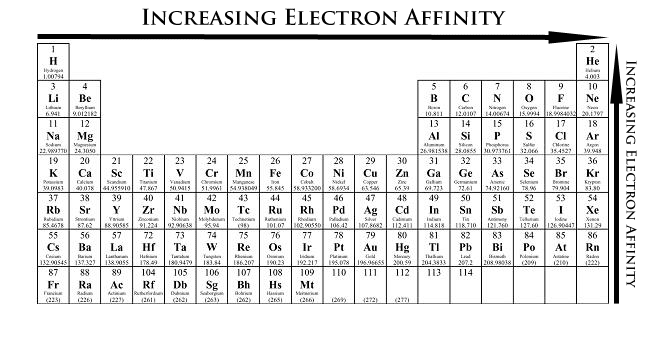
Trends in Electron Analogousness
Electronegativity
Electronegativity is the tendency of an atom to attract shared electrons to itself when it forms a chemical bond with some other atom. Ionization potential and electron affinities are regarded as more or less expressions of electronegativities. Atoms with small size, high ionization potential and high electron affinities would be expected to take high electronegativities Atoms with orbitals near filled with electrons will have college expected electronegativities than atoms with orbitals having few electrons.Non metals take higher electronegativities than metals. Metals are more of electron donors and non metals are electron acceptors. Electronegativity increases from left to correct within a period and decreases from top to bottom within a group.
Electronegativity and Periodic Tabular array
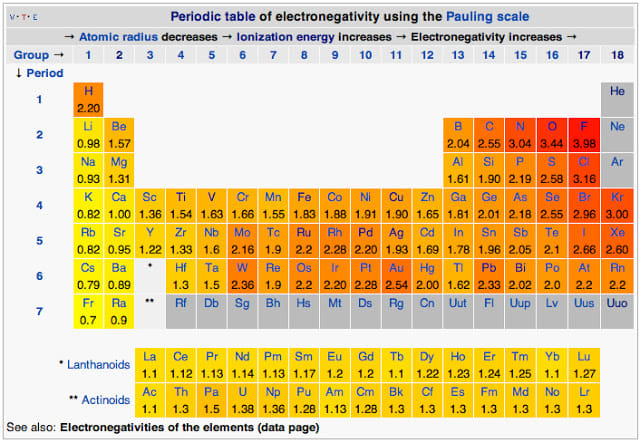
Electronegativity increases from left to correct inside a period and decreases from pinnacle to bottom within a group.
Summary of the Trends in the Periodic Tabular array
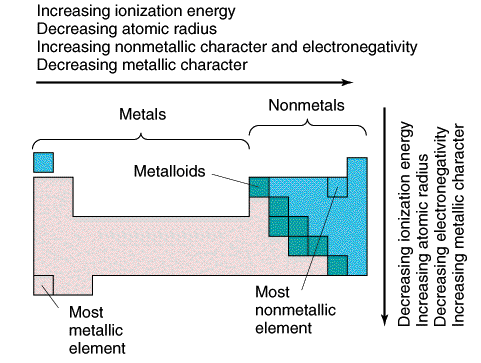
Readings on Periodic Table
- Periodic Properties of the Elements
Larn virtually the periodic properties or trends in the periodic table of the elements.
Video on Periodic Table
Self - Progress Test
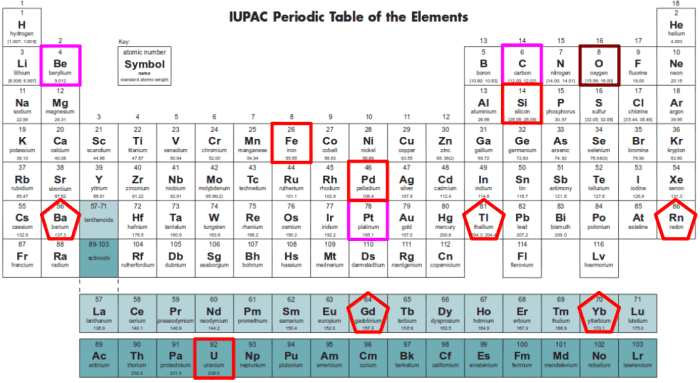
hypothetical Periodic Tabular array
A. I. Based on the given IUPAC Periodic Table and hypothetical elements equally positioned, respond the following:
i. The almost metallic element.
2. The nearly nonmetallic element.
3. The element with the biggest diminutive size.
four. The element/s classified every bit alkali metallic/s.
5. The element/southward classified every bit metalloids.
6. The element/s classified alkali-world metals.
7. The transition element/s.
viii. The element/due south classified every bit halogens.
9. The lightest of the noble gas.
10. Chemical element/south with electronic configuration/s ending in d.
11. Element/s with electronic configuration ending in f.
12. Element/s with ii (2) valence electrons.
13. Element/south with six (six) valence electrons.
14. Element/s with eight (eight) valence electrons.
15. Element/south with one main free energy level.
2. Reply fully the following questions:
1. Land the Periodic Law.
2. Explicate conspicuously what is meant by the statement that the maximum possible number of electrons in the outermost energy level is eight.
three. What are transition elements? How do y'all account for the marked differences in their backdrop?
B. Copy and fill up in the table below:
kalifa Ibrahim on November 30, 2019:
periodic table is the most important things in chemistry
Esther Nneli on September 20, 2019:
What is periodic law. And procedures for chemistry practicals
Abe Puzon from Bulacan, Philippines on July 12, 2018:
PUZON, ABEGAIL Southward. BSMT-1
Nosotros tin chosen the Periodic Table as the "cardinal of life" considering all the elements or compounds of the elements of on the periodic table make upward the earth. The periodic table of elements is a fabulous way to organize and empathize elements that brand upwardly everything. Chemical reactions and elements affect the fashion we live. The periodic table is organized then people tin can understand the properties of sure elements.
De Leon, Tune Jane on July 12, 2018:
For this topic, we learned that the periodic tabular array composed of dissimilar elements that is according to their atomic no., diminutive mass even the protons, electrons, electronic configuration and atomic construction.
Pauline Venzon on July 12, 2018:
Venzon, Pauline Charm Southward.
BSMT-i
The periodic table is the most important in chemistry reference there is . it arrenges all the know elements in an informative assortment
MIRANDA, MA. PINKY RAMEI V. (BSMT 1) on July 09, 2018:
As a Medtech student, we ever deal with chemical elements. And as a medical student it is important for united states of america to know what does that element composed of, how does it react with other elements, and what is the use of that element because in laboratory test, reagents are used to know the blood type of a person, is information technology positive or negative and besides in testing BUN and Creatinine and other tests to identify what does our torso lacks of or have an excess of.
BUNDALIAN, CHLOE JANE L. on July 09, 2018:
(BSMT 1)
The periodic table is useful for mod students and scientist and peculiarly for me (a medtech student). We should not forget what we had learned and we should be familiarized considering nosotros might use this in the near future.
Patricia Mae Chiliad. Sayco on July 09, 2018:
SAYCO. PATRICIA MAE M. BSMT1. Periodic table has big responsibleness in our life including the unlike elements that discovered by the scientist. Our body needs unlike elements to survive similar Oxygen, Hydrogen, Fe, Calcium and etc. and we release Carbon Dioxide when we breathe out. Periodic table is the most of import tool in chemistry because of its function and also of having atomic properties that are useful for making a study in laboratory.
Nhiza Mae Fajardo on July 08, 2018:
FAJARDO, NHIZA MAE A.
(BSMT-1)
-Periodic table of elements is very important because it gives u.s.a. the informations we have to know. Nosotros have to familiarize ourselves as well with this elements for united states of america to be able to know each. Periodic table of elements is a large assistance to u.s. because the mod one is organized. Every bit a medtech student, studying this should non be missed because more or less of these elements are related to our field.
Angelou Pascual on July 08, 2018:
PASCUAL, MARY ANGELOU C. (BSMT-1)
Every bit a medtech student, knowing and studying of periodic table is very of import to us because it can give united states of america a background about elements that we will encounter. Periodic table is a tool where yous can find and know the diminutive number, diminutive mass, groups and periods of an element. This lesson is big aid for us.
anjelacastro2 on July 07, 2018:
CASTRO, ANJELA V. (BSMT-1) – The periodic tabular array is one of the most important tools in the history of chemistry. It describes the atomic properties of every known chemical chemical element in a concise format, including the atomic number, atomic mass and relationships betwixt the elements. Information technology allows the students to study different elements also as their groups and periods
CONSON, JIMELLE - BSMT 1. on July 07, 2018:
tudying nigh Periodic Table enables me to get more information virtually elements and its properties. Fifty-fifty the classifications of elements. The elements that are considered as metals, nonmetals, and metalloids. I besides learned well-nigh the elements that are considered as the most-metallic, well-nigh-nonmetallic, the dissimilar groups and periods, etc.
Darah Arthria Cristobal from San Ildefonso, Bulacan on July 07, 2018:
CRISTOBAL, DARAH ARTHRIA DC. BSMT 1. Periodic table is organized like a large grid and each element is placed in a specific location considering of its atomic structure. Every bit with any grid, the periodic tabular array has rows (Families or Periods) and columns (Group). Each row and column has specific characteristics.
Joyce Nicole Dungo on July 07, 2018:
BSMT
Having knowledge about the periodic table is really a huge thing in our future profession. Past studying the periodic table, We can clearly identify information about the properties of each elements considering elements are bundled by order of their atomic number. Ane of the most useful feature of the periodic tabular array is information technology can provide all the data that we need during an experiment.
kathlenecalague on July 07, 2018:
BSMT ane
Studying the periodic table of elements was kinda hard at showtime but when you dig deeper into it, y'all will capeesh its importance. The elements that are included in the periodic table was very essential to every medical student like me. Because as a hereafter scientists, we volition deal with different elements in the laboratory. So we should accept a background and knowledge nearly its type and chemical reactions that are likely for an chemical element. This information provided by Prof. Liwayway was very helpful for us to understand it conspicuously.
Emman Payuran on July 07, 2018:
EMMANUEL VILLASENOR BSMT ane
Studying the periodic table with you Ma'am makes information technology a lot easier and fun because we're learning and at the same time we're laughing. And now I'm starting to beloved chemical science because of you ma'am.
So learning the periodic table, groups, families, valance electron etc. etc. I realized that all of the scientist that work together to build those elements to accommodate those elements in order, worked hard, for the states the time to come Medical Technician to learn the importance of it. It's very important to learn the periodic tabular array especially in our field but thanks to you prof! we are learning many things! Thank you!
Kimberly Reforsado on July 07, 2018:
BSMT-1
Knowledge near the Periodic Table, which gives u.s. significant information well-nigh different elements, is very essential as a futurity Medical Technologist because in the future, we are going to deal with different kind of elements. Having knowledge most them will arrive easier for united states to empathise them which is a huge factor in fulfilling the job that nosotros volition have in the future thus, emphasizing its relevance to our class.
Mary Elaine V. Herrera BSMT on July 07, 2018:
Knowledge about the periodic table of elements is important equally a future medical technologist. Knowing the elements will help me understand them when we see them in the laboratory. Understanding the periodic table of elements is really relevant as a before long to exist medical technologist.
Tuazon Allen Patrick BSMT on July 07, 2018:
This cognition volition help us for our future career and volition give us a brief groundwork about the periodic table and every elements office on information technology. Reading this commodity reminds me that periodic table is of import in our daily life by only knowing what elements you are using and what are the properties it contains. To the given elements in the periodic table nosotros can create and use that element in our daily life for our survival and other things.
Ma. Roselle Angela Due south. De Leon on July 07, 2018:
BSMT
Studying the periodic tabular array of elements is very much needed in a future Medical Technologist. It will gives us knowledge about different elements that nosotros will encounter in many labaratory experiments. Knowing the elements, its atomic mass, atomic number, electron, proton, neutron, valence, electronic configuration, groups and catamenia volition be a very big assistance to us to know their structures. This lesson is very relevant to my class which is Medical Technology.
Tricia Anne Reyes BSMT from Palapala, San Ildefonso Bulacan on July 06, 2018:
Tricia Anne Reyes BSMT
Having knowledge about the periodic table is 1 of the interesting thing that you'll have. Information technology involves what are the element/due south that has high electron negativity, depression electron negativity, loftier electron affinity, and high ionization potential. You can also learned what is the most metal element and most non metallic element, halogens over noble gases. With the help of Periodic Table of elements , the Atomic number is arranged increasingly which helps us to hands trace the elements.
KristineCassandra on July 06, 2018:
Jota, Kristine Cassandra B.
BSMT
As a futurity medical technologist, we need to know these things that tin give us a brief background about the periodic table of elements. Periodic table is a master slice because of organized elements' information, they are bundled clearly by their atomic number. Without the periodic table of elements, each element volition non exist unique if they have the same diminutive numbers.
Chan, John Derrick L. on July 03, 2018:
Knowing something virtually periodic table can give me a brief background nearly elements. In my hereafter workplace, we need to deal too with the xiii essential elements in our body and knowing something about those elements, we can auscultate more how it behaves thus, creating a more accurate understanding about those. As a future medical professional person, dealing with these things is our nature and truly, learning something nigh periodic table is a swell help.
CRUZ, BEATRIZ DL. BS PSY3 on April 24, 2018:
The periodic table is a tabular organisation of the chemical elements, ordered by their diminutive number, electron configuration, and recurring chemic properties, whose adopted construction shows periodic trends. Generally, within one row (period) the elements are metals on the left, and non-metals on the right, with the elements having similar chemic behaviours being placed in the aforementioned column. Table rows are commonly called periods and columns are called groups. Vi groups have accepted names also as assigned numbers: for case, grouping 17 elements are halogens; and group xviii are noble gases. Also displayed are 4 unproblematic rectangular areas or blocks associated with the filling of unlike diminutive orbitals.
John Lawrence Delos Santos on April 24, 2018:
Summer Tutorial In Chemisry ever employ the periodic table , it was discovered by Dmitri Mendeleyeev (1869)
He worked out a Periodic Table of Elements were the elements were bundled in the order of increasing atomic weights with a regular repetition (periodicity) of physical and chemic properties. In this table we can see all the elements , we can besides written report different parts of periodic table it shows the electrical configuration , or the explanation of each parts or nosotros can tin can also see the atomic number , atomic mass , the proton neutron electron of each elements
ivoryshanesalonga on Jan 18, 2018:
ivory shane f. salonga
bstm 4
(tutorial/friday)
the periodic table of the elements of the elements has gone through numerous revisions over the years as scientists have gained more noesis nearly the atomic structure of the elements. The most recent version of the periodic table provides useful information that, directly or indirectly, affects anybody. some people didnt know the importance of this merely, they should know about this.
Jerielen Mangahas on January 09, 2018:
Jerie Len Due south. Mangahas
BSTM4
(Fri-TUTORIAL)
Periodic Table is of import in chemistry because for studying elements in a systematic and standard manner.
Ronniel Legaspi on July 14, 2017:
RONNIEL AERON R. LEGASPI BSEEC-3
The periodic table organizes elements according to like backdrop then you tin can tell the characteristics of an element just past looking at its position on the table and to distinguish what element nosotros need to apply in our lab.
Javy Paul Gonzales from San Ildefonso Bulacan on July 14, 2017:
GONZALES, JAVY PAUL South. BSHM3
Periodic table is very relevance to hospitality Management considering information technology will give the states an edge to distinguished nonmetal to metal and also we can know what possible element nosotros can use to give our job an extra level like liquid nitrogen at present use in cooking so periodic tabular array is relevant to u.s..
GONZALES, JAVY PAUL S. BSHM3 on July 14, 2017:
Periodic table is very relevance to hospitality Management considering it will give u.s.a. an edge to distinguished nonmetal to metal and too we can know what possible chemical element we can employ to give our job an extra level like liquid nitrogen now use in cooking and so periodic tabular array is relevant to usa.
MJ Melencio on July 14, 2017:
Marc James Melencio
Tourism
Full general Chemistry
T/F seven:30AM-12:00PM
The periodic table is a tabular organization of the chemical elements, ordered by their atomic number (number of protons), electron configurations, and recurring chemical properties. This ordering shows periodic trends, such every bit elements with like behaviour in the same cavalcade. It also shows four rectangular blocks with some approximately similar chemical properties
Marc James Melencio on July 14, 2017:
Tourism
General chemistry
T/F 7:30am-12:00pm
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons), electron configurations, and recurring chemic properties. This ordering shows periodic trends, such as elements with similar behaviour in the same column. Information technology besides shows iv rectangular blocks with some approximately similar chemic backdrop
Jasper Sierto on July 13, 2017:
Sierto Jasper L.
The periodic tabular array is a tabular arragement of the chemical elements, ordered bt their atomic number number (number of protons), electron configurations, and recurring chemical properties.
As a Hospitality Management, we demand to know this considering we're on the service industry. We need to prioritize the safety of our customer
kenneth sanciangc on July 13, 2017:
Sanciangco Kenneth Philip H. BSHM
The periodic table is organized like a guess in a hotel each elements is placed in a specific location or rooms because of its atomic construction. Each rows and column has specific characteristics similar the different types of hotel rooms like single room,double room, queen bed etc. that may offer to the guest.
Rene Briane on July 13, 2017:
Rene Briane G. Angeles
BSEEC-III
Periodic table of elements are very important to us all bacause it can be use in all things. to know all the elements that is existing in the world.
Information technology is helpful for engineers to know what is the best element that they can use by using periodic table of elements.
Christine San Gabriel BSN1 on July thirteen, 2017:
Periodic table is a table that show example of chemicals grouped together to understand its composition,and help united states derive relationships betwixt the elements.
Danica Vipinoza on July 12, 2017:
For me, every bit a Hospitality Direction Educatee information technology is important for u.s.a. to know the different elements and and nosotros can predict the types of chemical reactions that are probable for an element. We can work safely if we know well-nigh those components of those materials so we tin properly use the materials we needed in our grade.
Camille San Mateo on July 12, 2017:
San Mateo, Camille C.
BSN-i
The periodic table organizeelement according to similar properties then you can tell the characteristics of an element just by looking at its position on the table. Those elements are important because nosotros're surrounded by elements so that nosotros need to know something backside of information technology. We need to know at least some of these specially the xiii elements in a human body; hydrogen, carbon, nitrogen, oxygen, sodium, magnesium, phosphorus, sulfur, chlorine, potassium, calcium, iron, iodine.
Jolina Gabriel on July 12, 2017:
JOLINA GABRIEL
BSN-1
the periodic table organizes elements to make nursing student easier to notice. nurses might need them to know what drugs to employ and students use them every bit a ways of system. its much easier to find the diminutive mass of carbon by looking it upwardly on the tabular array as opposed to looking it up in a textbook.
Dave Joshua Villangca on July 07, 2017:
Dave Joshua B. Villangca
BSN-I
Baliuag Academy
General Chemistry
T, F 7:30-12:00
The Periodic Table is a tabular form wherein the elements are organized based on their atomic numbers and electron configuration. Every bit a nursing student, it is of import to know, memorize, and sympathize the 13 essential elements plant in the Human Torso- which are the carbon, hydrogen, oxygen, phosphorus, potassium, iodine, nitrogen, sulfur, calcium, iron, magnesium, sodium, and chlorine- in lodge for me to empathise more how they function within our body for us to become healthy and how we get disease when the elements are not in their usual set points. Understanding these elements can be a primal to a door leading to a healthy and proper lifestyle.
TORIBIO, PAULINE LOUISE A. on July 07, 2017:
TORIBIO, PAULINE LOUISE A.
BSN1
The Periodic Tabular array is an organized list of elements. Everything around the states is made up of chemicals. So basically we should know the importance of these elements in social club for us to improve living.
Learning the periodic tabular array may not be easy but this is very applicable in every person and one's living.
For a nursing student like me, the deeper explanation of elements such every bit their structure is worthy. The 13 basic components of the homo body, their reactions to other elements or mixtures may help me discover or empathise how a human body functions.
Bianca Bernardo on July 04, 2017:
Marucot, Katrina Bianca BSN 1 GEN CHEM 2017
Understanding the periodic table is non only essential to the report of Nursing but is also very helpful. This serves as a guide to understanding dissimilar chemicals but the periodic table as well shows us the elements dissimilar properties and how to properly combine them and use them. Through the periodic table, we meliorate understand the essential elements in our daily lives such as Carbon, Oxygen, Hydrogen, Iron, Magnesium, Calcium, Phosporus, Zinc and many more which are essential to our bodies. The periodic table helps u.s. farther sympathise how to chemically combine different elements and how to split up them properly.
RussellBernardino on July 03, 2017:
RUSSELL BERNARDINO BSTM4. Periodic tabular array helps provide , students like me, with an invaluable tool to apace predict an chemical element'due south backdrop, it's atomic mass, atomic number and what group it belongs to.
Jahna Carmella B. Galvez on February 25, 2017:
Jahna Carmella B. Galvez
BEED-II
There are many reasons why the Periodic Table of Elements is important. The first and most important perhaps is that the elements are arranged past society of atomic number which allows scientists to clearly place the backdrop of the elements. And too the periodic table organizes elements co-ordinate to similar properties and so y'all tin can tell the characteristics of an element merely by looking at its position on the tabular array. And every bit a future educator nosotros should larn how to organize the course properly and systematically.
Salvador, Jucel Roma One thousand. on Feb 25, 2017:
The periodic table is the most important chemistry reference. Elements are bundled left to right and top to bottom in order of increasing atomic number. Information technology generally coincides with increasing atomic mass. Using the data in the periodic tabular array, students and others that are familiar with the periodic tabular array can extract information apropos individual elements. Students also proceeds information from the periodic table past looking at how it is put together. Past examining it, they will also exist aware of the dissimilar elements and their groupings or classifications.
JosephineLopez04 on February 25, 2017:
Josephine Five. Lopez BEEd - Two
The periodic table is a tabular organisation of the chemical elements, ordered by their atomic number (number of protons), electron configurations, and recurring chemical properties. This ordering shows periodic trends, such as elements with similar behaviour in the same column. Information technology as well shows four rectangular blocks with some approximately similar chemical properties. In general, within one row (period) the elements are metals on the left, and non-metals on the right.
The rows of the tabular array are called periods; the columns are called groups. Six groups have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships betwixt the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behaviour, and is widely used in chemistry and other sciences.
Cristina Perez on February 25, 2017:
Perez, Ma. Cristina Grand.
BEED-II
CAS 105/ tf/ 12:30-2:00
The first and most important perhaps is that the elements are arranged by order of atomic number which allows scientists to clearly place the backdrop of the elements. Scientific researchers agree that the periodic table is a master piece of organized chemic information.
The periodic table is useful considering it identifies and arranges all known elements in an informative style. Elements are arranged into periods and families. The elements in each family or flow may have like or different concrete and chemical backdrop.
The periodic table arranges the elements into families and periods (vertical and horizontal rows). The elements in each family unit have similar properties. ... The table tells you what elements may accept similar chemical and physical properties. The periodic table describes the diminutive construction of all known elements.
Carmela Perez on February 25, 2017:
Perez, Ma. Carmela M.
BEED-II
(TF/ 12:30-two:00/ CAS 105)
In that location are many reasons why the Periodic Table of Elements is of import. The showtime and well-nigh important perchance is that the elements are arranged by order of atomic number which allows scientists to clearly identify the properties of the elements. Scientific researchers hold that the periodic table is a master piece of organized chemical information. The progression of the periodic table into its current course is a major achievement in and of itself with contributions from many famous pharmacist and scientist. The mod periodic table has inverse since Mendeleev's original table, nonetheless both the get-go tables and the modern table are important for the aforementioned reason: The periodic table organizes elements co-ordinate to similar backdrop so you can tell the characteristics of an chemical element just past looking at its position on the table. he table is useful for modern students and scientists because it helps predict the types of chemical reactions that are probable for an element. Rather than memorize facts and figures for each element, a quick glance at the table reveals a lot almost the reactivity of an element, whether it is likely to conduct electricity, whether it is hard or soft, and many other characteristics.
Villaroman, Nikki D.C. BEEd III on February 25, 2017:
Periodic table of the elements, in chemistry, the organized assortment of all the chemical elements in order of increasing diminutive number—i.east., the full number of protons in the atomic nucleus. When the chemical elements are thus arranged, at that place is a recurring pattern called the "periodic police force" in their properties, in which elements in the aforementioned cavalcade (group) take like properties. (See Figure 1.) The initial discovery, which was made by Dmitry I. Mendeleyev in the mid-19th century, has been of inestimable value in the development of chemistry.
Information technology was not actually recognized until the second decade of the 20th century that the society of elements in the periodic system is that of their atomic numbers, the integers of which are equal to the positive electrical charges of the atomic nuclei expressed in electronic units. In subsequent years corking progress was made in explaining the periodic police in terms of the electronic construction of atoms and molecules. This description has increased the value of the law, which is used equally much today every bit information technology was at the start of the 20th century, when it expressed the only known human relationship amidst the elements.
Maniella de Leon on Feb 24, 2017:
Periodic table is the most important tool in studying chemistry. It is the middle of chemistry. Without this, amend understanding of chemistry would exist impossible. or in that location would be no learning at all.
In relation with teaching profession, The elements in the periodic tabular array are our students. Teachers must know them well, know the capabilities of every student. because in teaching, the heart of information technology belongs to the students.
mariseperez08 on February 24, 2017:
Perez, Nina Marise 5.
BEED-II
TF-12:30-2:00pm/CAS 105
The periodic table is a tabular organization of the chemical elements, ordered past their atomic number (number of protons), electron configurations, and recurring chemical properties. This ordering shows periodic trends, such equally elements with similar behaviour in the aforementioned column. Information technology besides shows four rectangular blocks with some approximately similar chemical properties. In general, within one row (period) the elements are metals on the left, and not-metals on the right.
The rows of the table are chosen periods; the columns are called groups. Vi groups have names every bit well as numbers: for example, group 17 elements are the halogens; and grouping 18, the noble gases. The periodic table tin can exist used to derive relationships between the properties of the elements, and predict the backdrop of new elements yet to exist discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behaviour, and is widely used in chemistry and other sciences.
The Russian pharmacist Dmitri Mendeleev published the first widely recognized periodic table in 1869. He developed his table to illustrate periodic trends in the properties of the and then-known elements. Mendeleev likewise predicted some backdrop of then-unknown elements that would exist expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev'due south periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the evolution of new theoretical models to explain chemical behaviour.
All elements from atomic numbers 1 (hydrogen) to 118 (oganesson) take been discovered or synthesized, with the virtually recent additions (nihonium, moscovium, tennessine, and oganesson) existence confirmed past the IUPAC on December 30, 2015: they complete the first seven rows of the periodic tabular array.[1][2] The first 94 elements be naturally, although some are found only in trace amounts and were synthesized in laboratories earlier being establish in nature.[northward i] Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories or nuclear reactors.[iii] Synthesis of elements having higher atomic numbers is being pursued. Numerous constructed radionuclides of naturally occurring elements accept also been produced in laboratories.
Maria Luz Mendoza on Feb 24, 2017:
MA. LUZ MENDOZA BEED II
The Periodic Table of the Elements arranges all of the known elements in an informative array. Elements are arranged in order of increasing atomic number. Order generally coincides with increasing atomic mass. The periodic table is useful because it identifies and arranges all known elements in an informative way. Elements are arranged into periods and families. The elements in each family or period may take similar or dissimilar physical and chemical properties. The periodic table is the almost important chemistry reference there is. It arranges all the known elements in an informative assortment. Elements are arranged left to right and top to lesser in order of increasing diminutive number. Order generally coincides with increasing diminutive mass. The elements on the periodic table is so important in life today, it has helped scientist notice so many big events. The periodic table helped scientist discover what elements were needed for humans to survive. The elements also aid u.s.a. detect our resources and what they need. Similar plants and Animals demand elements to grow. Similar chlorine it helps animals assimilate food and humans, atomic number 26 helps blood carry oxygen around the body and magnesium and chlorine to help plants convert the suns energy to food. Grass contains silica that makes it very potent. And then, without discovery of the elements on the periodic table people wouldn't know how to survive, there would be no healthy foods and coin would not exist in life.
Elize Bantang 14 on February 24, 2017:
Eliza B. Merle BEEd-ll
Periodic table of elements is the near of import chemical science refence. Information technology arranges all the known elements in an informative assortment. Elements are arranged left to right and top to lesser in order of increasing diminutive number and coinsides with increasing atomic mass. The importance of the periodic tabular array is that it provides a fashion of organizing the elements so that it's possible to make certain predictions well-nigh their chemical and physical properties based on their position in the table. With the assist of periodic table, nosotros tin can learn the backdrop of many elements at a time and classification of elements volition be easier. For e.grand. group 1A elements are metal, then we can figure out that all 7 elements i.e Hydrogen, Lithium, Sodium, Potassium, Rubidium, Cesium and Fracium are electro-positive in nature.
Karen Manalac, BAC IV 7:30-12:00 on Dec 08, 2016:
The periodic table of elements is a tool that can be useful in such ways. Present in a periodic table of elements are the elements that are discovered and that be in this world. Information technology is important to be familiarize with that so that when there comes a time when you kind of encounter some elements, you may know a little bit of a background to that particular chemical element. It it meaning to our form due to the fact that nosotros, researchers need to ever dig on information that will exist given to the people.
Chella Maniquiz, BSTM 7:30-12:00 on December 07, 2016:
The periodic table is the most useful tool in chemistry. The periodic table was used to predict all the element on the table. It helps usa know its diminutive number and its diminutive weight. Periodic table is also useful in our course Tourism or in Hospitality Management because it keep u.s. to exist aware in knowing what kind of elements when we prefer to work every bit a housekeeper in the future.
Nixie Miranda BAC II on December 05, 2016:
Periodic table is the most important chemistry reference. It arranges all the known elements in an informative assortment. Elements are bundled left to correct and top to lesser in gild of increasing diminutive number. Order mostly coincides with increasing atomic mass. The Periodic Law states that the physical and chemic backdrop of the elements recur in a systematic and anticipated way when the elements are arranged in order of increasing atomic number.
Mary joy Anoche BSA 1-2 on Jan 02, 2016:
periodic tabular array is non about presenting the proper noun of the element, it show the atomic no or what that chemical element consist, and there is an sectionalisation with it. periodic table is important to us to learn what element is needed in chemical like the h2o its consist of ii hydrogen and an oxygen.
John Ismael Espino Lozano from Baliuag Bulacan Philippines on December xix, 2015:
Lozano, John Ismael E. (BSA 1-2)
For me periodic table is the listing of all elements. Its is of import because our torso, everything, the world is made up of element. It has all the information of elements that needed on studies. It will be good if we all know each element in the periodic table
Kimberly Anne DC Magtalas on December 06, 2015:
Kimberly Anne DC. Magtalas BSA 1-3
The periodic table of elements is important in science because everything is made upwardly of elements. The periodic table is organized according to trends in element properties. The periodic table provides all the information you demand to remainder chemical reactions at a glance. It helps us predict the types of chemical reactions that are likely for an element. We can learn the properties of many elements at a time and classification of elements volition be easier.
Rolaine Ann Ingalla on December 01, 2015:
BSA 1-3 Ingalla, Rolaine Ann B.
Rolaine Ann Ingalla on December 01, 2015:
A periodic tabular array, a listing of chemical elements arranged co-ordinate to their backdrop, can be similar to a chart of accounts, a listing of all accounts that are accompanied by their reference number, which can help the users to easy locate a specific element or an business relationship title. Having enough noesis on how the elements are located to their respective position now and how it will immediately locate can aid u.s. to be fourth dimension efficient and, therefore, more works to finish and more pay in return.
Genierose de Ocera from San Miguel, Bulacan on November 20, 2015:
Genierose de Ocera
BSA I-4
The periodic table of the elements has gone through numerous revisions over the years as scientists have gained more knowledge nigh the diminutive structure of the elements. The well-nigh recent version of the periodic tabular array provides useful information that, straight or indirectly, affects anybody.The periodic tabular array is ane of the most meaning achievements in science, capturing the essence not only of chemical science but also of physics and biological science. Information technology is a unique tool, enabling scientists to predict the advent and properties of matter on Earth and in the residue of the Universe. It gives a systematic business relationship of the early developments that led to the classification of the elements. Every bit an accountancy student who is taking up Science subjects, the table is useful for us including the scientists because it helps predict the types of chemical reactions that are probable for an element. Rather than memorize facts and figures for each element, a quick glance at the table reveals a lot most the re activity of an element, whether it is likely to bear electricity, whether information technology is difficult or soft, and many other characteristics.
Angelica Salinas on November 14, 2015:
Angelica G. Salinas BSBA 1-1 - The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical backdrop. The table likewise shows 4 rectangular blocks: due south-, p- d- and f-block. In general, within 1 row (period) the elements are metals on the lefthand side, and non-metals on the righthand side. The rows of the tabular array are called periods; the columns are called groups. Half-dozen groups (columns) have names as well equally numbers: for example, group 17 elements are the halogens; and grouping 18, the noble gases. Dmitri Ivanovich Mendeleev was the one who formulated the Periodic Police, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and likewise to predict the backdrop of eight elements yet to be discovered.
Paulo Tomas on March 10, 2015:
TOMAS J P BUHM
A periodic table can help us to see all of the chemical symbol, mass, units etc. It can help the states to written report the chemical in easy way and it like a complete package you can encounter the definition of a specific chemical
Jole Ymenel Tila from Pulilan, Bulacan on March 09, 2015:
Tila Jole Ymenel F. BUHM
The relevance of periodic table in Hospitality Management is information technology keeps u.s.a. aware in knowing what kind of element is compatible to another chemic chemical element that would give satisfaction for a better product.
Rad Mesina from Calumpit, Bulacan on March 05, 2015:
23 MESINA, DJ BUHM
The table is useful for modern students like u.s.a. considering it helps predict the types of chemical reactions that are likely for an element. Rather than memorize facts and figures for each element, a quick glance at the table reveals a lot almost the reactivity of an element, whether information technology is likely to deport electricity, whether information technology is difficult or soft, and many other characteristics.
jairafulgencio on March 05, 2015:
FULGENCIO JM BUTM 2014
It is important fro us to know how meaning the periodic tabular array in our course. As I take my ain thought, for me, it is really of import for us because we should know the components of every equipment and materials nosotros are going to employ
28quizon,CA on March 05, 2015:
as a tourism student it is very important to know the elements and components of periodic table so that we can understand what utilise of that element and substance.
Zyrine Mae Taruc on March 05, 2015:
Taruc, ZD, BUTM 2014
Product of elements having higher atomic numbers is being pursued, with the question of how the periodic table may need to exist modified to accommodate any such additions being a matter of ongoing debate.
MARCELO JME BUTM on March 05, 2015:
The periodic table is the well-nigh important chemistry reference there is. It arranges all the known elements in an informative assortment. Elements are arranged left to right and top to bottom in guild of increasing atomic number. Lodge generally coincides with increasing atomic mass.
Janice Gonzales on March 05, 2015:
GONZALES JB BUTM
The of import of periodic table is useful for modern students and scientists considering it helps predict the types of chemic reactions that are probable for an element.
Mary Rose Banzon on March 05, 2015:
Periodic table was a main view in chemical science. When i was high school my teacher assigned us to memorized periodic tabular array and now it is useful in my industry because of my gen. chemical science discipline.
Mary Rose Banzon on March 05, 2015:
Existence a TM student and an gen. chemistry student of prof liway the periodic table is of import. even in high schoolhouse our teacher is assigning united states to memorized the periodic tabular array and right now i remember it is helpful in my subject.
Marife DG. Montejo on March 05, 2015:
The importance of studying and learning well-nigh the different elements in periodic table for united states of america is to identify what elements is being used to create a certain matter. And what element is nowadays on the thing we may used in the future to our job.
MarjorieLansanganBSHM on March 04, 2015:
The relevance of periodic table to us is to know how it was formed how is information technology arrange considering in Hospitality Management we will handle several things that composes an element it is relevant because it will give the states an edge to distinguished not metal to metal
19 Garcia R. BUHM on March 04, 2015:
A periodic tables are important to every bit because they tin can help to us to see all of the chemical symbol,mass,units etc. Being a HM students it is very important to know the elements and components of periodic tables.
Steph San on March 04, 2015:
30SANTIAGO S, BUHM
The periodic tabular array of the elements has gone through numerous revisions over the years as scientists accept gained more knowledge about the atomic structure of the elements. And periodic table assistance usa to know what are the difference betwixt all those chemical element and information technology assistance united states when preparing food because information technology teach us the differences betwixt chemicals
Erwin pascual on March 04, 2015:
A periodic tabular array tin help united states of america to see all of the chemical symbol, mass, units etc. It tin help us to report the chemical in easy way and it like a complete bundle you lot can see the definition of a specific chemical
john angelo southward. ortega bshm2 on March 04, 2015:
equally an hm student, information technology was very much important to know considering in my manufacture, i need to know the different backdrop and substances of the chemicals we are using in cleaning specific part of the hotel . also equally a cook, i need to know the different chemicals used in preparing or in making specific ingredients to make food.
Patrisha Anne D.Vanguardia from San Ildefonso,Bulacan on March 04, 2015:
For HM student it is very important to know the elements and components of periodic tabular array and then that nosotros tin can understand what employ of that element and substance.
John Andrew Cabrera on March 04, 2015:
The periodic table is useful for modern students and scientists because information technology helps predict the types of chemic reactions that are probable for an element.
Glenric Samaniego on March 04, 2015:
This helps us sympathise and know the true nature of elements that nosotros utilize everyday. Many of us does non know that we use elements every bit part of our daily living.
John Carlo Viola on March 04, 2015:
The relevance of the periodic table to HM students, information technology may help u.s. and guide us to know what elements or substance we may use in cooking
Ellen Mae M. Canoza from Bonga Mayor, Bustos Bulacan on March 04, 2015:
03, Canoza EM BUHM
The relevance of Periodic Table in Hm students is a big telescopic for us,It's human nature to organize things. Cooks painstakingly organize their spices into various groupings, whether alphabetically or according to how often they're used. Kids dump out their piggy banks and sort their riches into piles of pennies, nickels, dimes and quarters. Even the items in a grocery store are grouped a certain way.
Eimon Paolo on March 04, 2015:
Periodic table is very of import considering information technology is our guide in chemistry.
simplyariannejoy on March 04, 2015:
17 Flores, AJ BUHM
The relevance of periodic table in hospitality management is information technology keeps us aware in knowing what kind of chemic/element is uniform to some other chemical/element that would requite satisfaction for a better product.
renzocauzon on March 04, 2015:
09 Cauzon,RA BUHM
The periodic table is the nigh important chemical science reference there is. The periodic tabular array is useful for modern students and scientists considering it helps predict the types of chemical reactions that are probable for an element.
Rolyn Santos from Bustos, Bulacan on March 04, 2015:
The periodic table is the most of import chemistry reference there is. It arranges all the known elements is an inforamtive array. elements are arranged left to right and peak to lesser is lodge of increasing atomis number. Club generally coincides with increasing diminutive mass.
Richmond V from Bustos, Bulacan on March 04, 2015:
The periodic table is very important for students because it tin assist us on learning the basic characteristics of an element and the reactions it can create with other elements.
Krisette Enriquez from Plaridel, Bulacan on March 04, 2015:
xiii ENRIQUEZ, KC BUTM 2014
We, Tourism students, besides demand to acquire about the Periodic table. It is helpful to identify a item substance once we already know something nearly a certain element.
Patricia Rivera on March 04, 2015:
In our example Periodic Tabular array was really non my type in chemistry because it has many to study with, to be honest. Merely I really understand how to use information technology and the importance of it. In tourism direction it can also be applied to my course and it helped me also past knowing the elements of it.
Assessment Capulong on March 03, 2015:
It'south a must to exist able to understand and how, especially, apply the periodic table. To specify the kind of elements. I recall when I was in high school the first element I learn is the Ca which is Calcium, well as for the other lessons periodic table is really useful for example: How to seek the diminutive orbitals and such In total, things that we use, swallow and see are things which involves the elements in the periodic tabular array.
kennethCorpuz on March 03, 2015:
periodic table is a powerful and connect of the course because tourism management is needed of symbol nunber and names of periodic like carbon and oxygen is important of animals and palnts. about scientist is learn of cholesterol from animals and plants is phytosterol..
AlmiraEvangelista on March 03, 2015:
Evangelista, Almira
BSTM l-fifty
When I first saw the periodic table, I had learn to apply the periodic table at the hard style. I wasn't sure how much it would help me, but when i started learning it, information technology fabricated me realized that the elements in periodic table take much important in my field.
AndreaBarcita on March 03, 2015:
3 BARCITA AR Thousand. BUHM14
The relevance of periodic tabular array in our course is when nosotros are cooking we cheque the ingredient if it is a liquid gas or solid. like in periodic table we cheque if its metal, metalloids or not metals and the different elements of this. Information technology is of import for us to know because if nosotros don't know, we might alter something to that chemical or nutrient
gelo hernandez on March 03, 2015:
Hernandez Angelo BSHM
Periodic tabular array is important considering of it we volition know the kinds of all metals, nonmetals and metalloids and as well we tin can determine if it tin can harm united states of america or not.
AndreaBarcita on March 03, 2015:
3 BARCITA AR Chiliad. BUHM14
The relevance of periodic table in our course is when we are cooking nosotros check the ingredient if it is a liquid gas or solid. Similar in periodic table we check if its metal, metalloids or non metals and the unlike elements of this. It is important for united states to know considering if we don't know, we might modify something to that chemical or food.
Cea Castillo on March 02, 2015:
The periodic table is useful for modernistic students and scientists because it helps predict the types of chemical reactions that are likely for an element. Rather than memorize facts and figures for each chemical element, a quick glance at the table reveals a lot thing that you lot need.
Brillant Joshua Dela Cruz on March 02, 2015:
periodic table is the most important in science.
Brillant Joshua Dela Cruz on March 02, 2015:
periodic table is the virtually important in scientific discipline.
robinsonwirave1956.blogspot.com
Source: https://owlcation.com/stem/The-Wonders-of-the-Periodic-Table
إرسال تعليق for "The Families of Instruments Are Arranged From Top to Bottom in Order of"